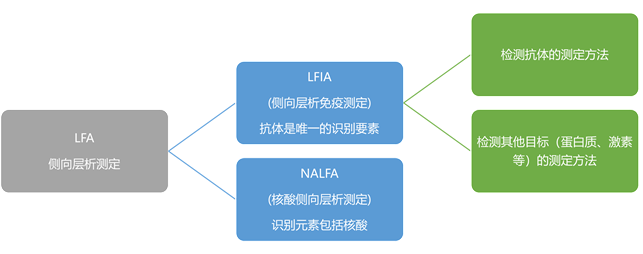
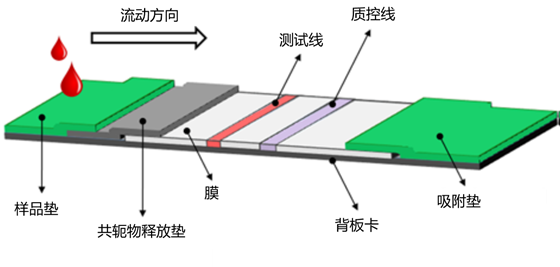
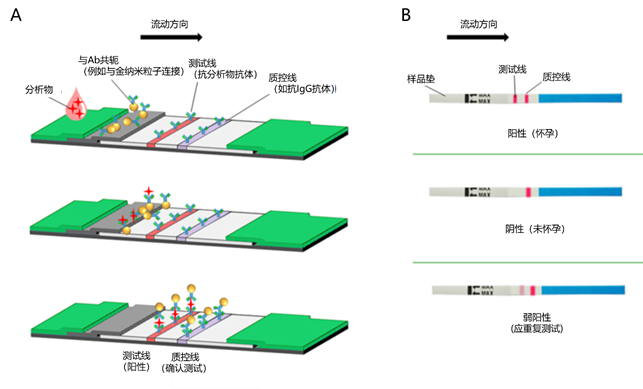
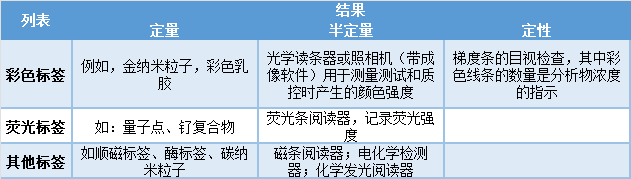
参考文献
[1] Boisen, M.L., Oottamasathien, D., Jones, A.B., Millett, M.M., Nelson, D.S., Bornholdt, Z.A. et al. (2015) Development of prototype filovirus recombinant antigen immunoassays. J. Infect. Dis. 212 (Suppl. 2), S359–367 CrossRef PubMed
[2] Nielsen, K., Yu, W.L., Kelly, L., Bermudez, R., Renteria, T., Dajer, A. et al. (2008) Development of a lateral flow assay for rapid detection of bovine antibody to Anaplasma marginale. J. Immunoassay Immunochem. 29, 10–18 CrossRef PubMed
[3] Rohrman, B.A., Leautaud, V., Molyneux, E. and Richards–Kortum, R.R. (2012) A lateral flow assay for quantitative detection of amplified HIV-1 RNA. PLoS One 7, e45611 CrossRef PubMed
[4] Kamphee, H., Chaiprasert, A., Prammananan, T., Wiriyachaiporn, N., Kanchanatavee, A. and Dharakul, T. (2015) Rapid molecular detection of multidrug-resistant tuberculosis by PCR-nucleic acid lateral flow immunoassay. PLos One 10, e0137791 CrossRef PubMed
[5] Moreno, M.L., Cebolla, A., Munoz-Suano, A., Carrillo–Carrion, C., Comino, I., Pizarro, A. et al. (2015) Detection of gluten immunogenic peptides inthe urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut, doi:10.1136/gutjnl-2015-310148
[6] Carrio, A., Sampedro, C., Sanchez-Lopez, J.L., Pimienta, M. and Campoy, P. (2015) Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors 15, 29569–29593 CrossRef PubMed
[7] Pacifici, R., Farre, M., Pichini, S., Ortuno, J., Roset, P.N., Zuccaro, P. et al. (2001) Sweat testing of MDMA with the Drugwipe analytical device: a controlled study with two volunteers. J. Anal. Toxicol. 25, 144–146 CrossRef PubMed
[8] De Giovanni, N. and Fucci, N. (2013) The current status of sweat testing for drugs of abuse: a review. Curr. Med. Chem. 20, 545–561 PubMed
[9] Magambo, K.A., Kalluvya, S.E., Kapoor, S.W., Seni, J., Chofle, A.A., Fitzgerald, D.W. et al. (2014) Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV-positive outpatients beginning antiretroviral therapy in Mwanza, Tanzania. J. Int. AIDS Soc. 17, 19040 CrossRef PubMed
[10] Schramm, E.C., Staten, N.R., Zhang, Z., Bruce, S.S., Kellner, C., Atkinson, J.P. et al. (2015) A quantitative lateral flow assay to detect complement activation in blood. Anal. Biochem. 477, 78–85 CrossRef PubMed
[11] Ang, S.H., Rambeli, M., Thevarajah, T.M., Alias, Y.B. and Khor, S.M. (2015) Quantitative, single-step dual measurement of hemoglobin A1c and total hemoglobin in human whole blood using a gold sandwich immunochromatographic assay for personalized medicine. Biosens. Bioelectron. 78, 187–193 CrossRef PubMed
[12] Nielsen, K., Yu, W.L., Kelly, L., Williams, J., Dajer, A., Gutierrez, E. et al. (2009) Validation and field assessment of a rapid lateral flow assay for detection of bovine antibody to Anaplasma marginale. J. Immunoassay Immunochem. 30, 313–321 CrossRef PubMed
[13] van Dam, G.J., de Dood, C.J., Lewis, M., Deelder, A.M., van Lieshout, L., Tanke, H.J. et al. (2013) A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. Parasitol. 135, 274–282 CrossRef PubMed
[14] Ching, K.H., He, X., Stanker, L.H., Lin, A.V., McGarvey, J.A. and Hnasko, R. (2015) Detection of shiga toxins by lateral flow assay. Toxins 7, 1163–1173 CrossRef PubMed
[15] Mei, Z., Qu, W., Deng, Y., Chu, H., Cao, J., Xue, F. et al. (2013) One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A (BPA) in aqueous samples. Biosens. Bioelectron. 49, 457–461 CrossRef PubMed
[16] Kim, Y.K., Lim, S.I., Cho, I.S., Cheong, K.M., Lee, E.J., Lee, S.O. et al. (2015) A novel diagnostic approach to detecting porcine epidemic diarrhea virus: the lateral immunochromatography assay. J. Virol. Methods 225,4–8 CrossRef
[17] Shukla, S., Leem, H., Lee, J.S. and Kim, M. (2014) Immunochromatographic strip assay for the rapid and sensitive detection of Salmonella Typhimurium in artificially contaminated tomato samples. Can. J. Microbiol. 60, 399–406 CrossRef PubMed
[18] Morales-Narvaez, E., Naghdi, T., Zor, E. and Merkoci, A. (2015) Photoluminescent lateral-flow immunoassay revealed by graphene oxide: highly sensitive paper-based pathogen detection. Anal. Chem. 87, 8573–8577 CrossRef PubMed
[19] Ngom, B., Guo, Y., Wang, X. and Bi, D. (2010) Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal. Bioanal. Chem. 397, 1113–1135 CrossRef PubMed
[20] Shyu, R.H., Shyu, H.F., Liu, H.W. and Tang, S.S. (2002) Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 40, 255–258 CrossRef PubMed
[21] Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L. and Xu, C. (2013) Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors 13, 4214–4224 CrossRef PubMed
[22] Lopez Marzo, A.M., Pons, J., Blake, D.A. and Merkoci, A. (2013) High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2 + detection in drinking waters. Biosens. Bioelectron. 47, 190–198 CrossRef PubMed
[23] Connelly, J.T., Nugen, S.R., Borejsza-Wysocki, W., Durst, R.A., Montagna, R.A. and Baeumner, A.J. (2008) Human pathogenic Cryptosporidium species bioanalytical detection method with single oocyst detection capability. Anal. Bioanal. Chem. 391, 487–495 CrossRef PubMed
[24] Xu, Y., Liu, Y., Wu, Y., Xia, X., Liao, Y. and Li, Q. (2014) Fluorescent probe-based lateral flow assay for multiplex nucleic acid detection. Anal. Chem. 86, 5611–5614 CrossRef PubMed
[25] Yen, C.W., de Puig, H., Tam, J.O., Gomez-Marquez, J., Bosch, I., Hamad-Schifferli, K. et al. (2015) Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing Dengue, yellow fever, and Ebola viruses. Lab Chip 15, 1638–1641 CrossRef PubMed
[26] Fung, K.K., Chan, C.P. and Renneberg, R. (2009) Development of enzyme-based bar code-style lateral-flow assay for hydrogen peroxide determination. Anal. Chim. Acta 634, 89–95 CrossRef PubMed
[27] Fang, C., Chen, Z., Li, L. and Xia, J. (2011) Barcode lateral flow immunochromatographic strip for prostate acid phosphatase determination. J. Pharm. Biomed. Anal. 56, 1035–1040 CrossRef PubMed
[28] Leung, W., Chan, C.P., Rainer, T.H., Ip, M., Cautherley, G.W. and Renneberg, R. (2008) InfectCheck CRP barcode-style lateral flow assay for semi-quantitative detection of C-reactive protein in distinguishing between bacterial and viral infections. J. Immunol. Methods 336, 30–36 CrossRef PubMed
[29] Reference deleted
[30] Workman, S., Wells, S.K., Pau, C.P., Owen, S.M., Dong, X.F., LaBorde, R. et al. (2009) Rapid detection of HIV-1 p24 antigen using magnetic immuno-chromatography (MICT). J. Virol. Methods 160, 14–21 CrossRef
[31] Butler, S.A., Khanlian, S.A. and Cole, L.A. (2001) Detection of early pregnancy forms of human chorionic gonadotropin by home pregnancy test devices. Clin. Chem. 47, 2131–2136 PubMed
[32] Mao, X., Ma, Y., Zhang, A., Zhang, L., Zeng, L. and Liu, G. (2009) Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal. Chem. 81, 1660–1668 CrossRef PubMed
[33] Parolo, C., de la Escosura-Muniz, A. and Merkoci, A. (2013) Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 40, 412–416 CrossRef PubMed
[34] Qiu, W., Xu, H., Takalkar, S., Gurung, A.S., Liu, B., Zheng, Y. et al. (2015) Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens. Bioelectron. 64, 367–372 CrossRef PubMed
[35] Ren, M., Xu, H., Huang, X., Kuang, M., Xiong, Y., Xu, H. et al. (2014) Immunochromatographic assay for ultrasensitive detection of aflatoxin B(1) in maize by highly luminescent quantum dot beads. ACS Appl. Mater. Interfaces. 6, 14215–14222 CrossRef PubMed
[36] Huang, C., Jones, B.J., Bivragh, M., Jans, K., Lagae, L. and Peumans, P. (2013) A capillary-driven microfluidic device for rapid DNA detection with extremely low sample consumption. 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013
[37] Anon (2008) Rapid Lateral Flow Test Strips: Considerations for Product Development, Merck Millipore, Billerica
[38] Anfossi, L., Di Nardo, F., Giovannoli, C., Passini, C. and Baggiani, C. (2013) Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal. Bioanal. Chem. 405, 9859–9867 CrossRef PubMed
[39] Lai, W., Tang, D., Que, X., Zhuang, J., Fu, L. and Chen, G. (2012) Enzyme-catalyzed silver deposition on irregular-shaped gold nanoparticles for electrochemical immunoassay of alpha-fetoprotein. Anal. Chim. Acta 755, 62–68 CrossRef PubMed
[40] Tang, D., Sauceda, J.C., Lin, Z., Ott, S., Basova, E., Goryacheva, I. et al. (2009) Magnetic nanogold microspheres-based lateral-flow immunodipstick for rapid detection of aflatoxin B2 in food. Biosens. Bioelectron. 25, 514–518 CrossRef PubMed
[41] Qin, Z., Chan, W.C., Boulware, D.R., Akkin, T., Butler, E.K. and Bischof, J.C. (2012) Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angew. Chem. Int. Ed. Engl. 51, 4358–4361 CrossRef
[42] Oku, Y., Kamiya, K., Kamiya, H., Shibahara, Y., Ii, T. and Uesaka, Y. (2001) Development of oligonucleotide lateral-flow immunoassay for multi-parameter detection. J. Immunol. Methods 258, 73–84 CrossRef PubMed
[43] Zhu, J., Zou, N., Mao, H., Wang, P., Zhu, D., Ji, H. et al. (2013) Evaluation of a modified lateral flow immunoassay for detection of high-sensitivity cardiac troponin I and myoglobin. Biosens. Bioelectron. 42, 522–525 CrossRef PubMed
[44] Chen, J., Fang, Z., Lie, P. and Zeng, L. (2012) Computational lateral flow biosensor for proteins and small molecules: a new class of strip logic gates. Anal. Chem. 84, 6321–6325 CrossRef PubMed
[45] Xu, H., Mao, X., Zeng, Q., Wang, S., Kawde, A.N. and Liu, G. (2009) Aptamer-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for protein analysis. Anal. Chem. 81, 669–675 CrossRef PubMed
[46] He, Y., Zhang, X., Zhang, S., Kris, M.K., Man, F.C., Kawde, A.N. et al. (2012) Visual detection of single-base mismatches in DNA using hairpin oligonucleotide with double-target DNA binding sequences and gold nanoparticles. Biosens. Bioelectron. 34, 37–43 CrossRef
[47] Liu, C., Jia, Q., Yang, C., Qiao, R., Jing, L., Wang, L. et al. (2011) Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal. Chem. 83, 6778–6784 CrossRef PubMed
[48] Li, Z., Wang, Y., Wang, J., Tang, Z., Pounds, J.G. and Lin, Y. (2010) Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem. 82, 7008–7014 CrossRef PubMed
[49] Joung, H.A., Oh, Y.K. and Kim, M.G. (2014) An automatic enzyme immunoassay based on a chemiluminescent lateral flow immunosensor. Biosens. Bioelectron. 53, 330–335 CrossRef PubMed
[50] Lin, Y.Y., Wang, J., Liu, G., Wu, H., Wai, C.M. and Lin, Y. (2008) A nanoparticle label/immunochromatographic electrochemical biosensor for rapid and sensitive detection of prostate-specific antigen. Biosens. Bioelectron. 23, 1659–1665 CrossRef PubMed
[51] Song, L.W., Wang, Y.B., Fang, L.L., Wu, Y., Yang, L., Chen, J.Y. et al. (2015) Rapid fluorescent lateral-flow immunoassay for hepatitis B virus genotyping. Anal. Chem. 87, 5173–5180 CrossRef PubMed
[52] Venkatraman, V. and Steckl, A.J. (2015) Integrated OLED as excitation light source in fluorescent lateral flow immunoassays. Biosens. Bioelectron. 74, 150–155 CrossRef PubMed
[53] Wang, D.B., Tian, B., Zhang, Z.P., Wang, X.Y., Fleming, J., Bi, L.J. et al. (2015) Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure”. Biosens. Bioelectron. 67, 608–614 CrossRef PubMed
[54] Mirasoli, M., Buragina, A., Dolci, L.S., Guardigli, M., Simoni, P., Montoya, A. et al. (2012) Development of a chemiluminescence-based quantitative lateral flow immunoassay for on-field detection of 2,4,6-trinitrotoluene. Anal. Chim. Acta 721, 167–172 CrossRef PubMed
[55] Maiolini, E., Ferri, E., Pitasi, A.L., Montoya, A., Di Giovanni, M., Errani, E. et al. (2014) Bisphenol A determination in baby bottles by chemiluminescence enzyme-linked immunosorbent assay, lateral flow immunoassay and liquid chromatography tandem mass spectrometry. Analyst 139, 318–324 CrossRef PubMed